The UK's medicines regulator has given approval for a groundbreaking gene therapy, Casgevy, to potentially treat two hereditary blood disorders.
This innovative treatment, designed for sickle cell disease and beta thalassemia, marks a significant milestone as the first licensed therapy using the CRISPR gene-editing tool, pioneered by Nobel Prize-winning inventors in 2020.
The tool that “edits” the gene that causes the blood disorders is also called the “genetic scissors,” The Guardian reported.
The Medicines and Healthcare products Regulatory Agency (MHRA) has granted authorisation for Casgevy to be used to treat sickle cell disease and beta thalassemia.
Sickle cell disease and beta thalassemia stem from genetic flaws affecting haemoglobin genes, critical for oxygen transportation by red blood cells throughout the body.
The developers of Casgevy envision that this pioneering treatment could alleviate the pain, infections, and anaemia associated with sickle cell disease, as well as address the severe anaemia commonly seen in individuals with beta thalassemia.
Approximately 15,000 individuals in the UK, predominantly of African or Caribbean descent, are living with sickle cell disease. Additionally, around 1,000 individuals, primarily from Mediterranean, south Asian, southeast Asian, and Middle Eastern backgrounds, are affected by beta thalassemia and require regular blood transfusions to manage their anemia.
People from south Asian communities are more likely to have conditions such as Thalassemia which also mean they need regular transfusions.
Experts in the field hold hope that Casgevy could offer a definitive cure, eliminating the need for bone marrow transplants, which have been the sole treatment option to date, despite the risk of donor marrow rejection.
The Sickle Cell Society hailed the MHRA's decision as a "historic moment for the sickle cell community," expressing renewed hope and optimism.
The charity further stated that the approval of Casgevy, developed by Vertex Pharmaceuticals, empowers the NHS to adopt it as a revolutionary therapeutic intervention for individuals battling this condition, characterised by chronic anemia, recurring episodes of excruciating pain necessitating hospitalisation, organ damage, an increased risk of stroke, and premature mortality.
Sickle cell disease and beta thalassemia are genetic disorders, or inherited conditions, arising from errors in the genes responsible for hemoglobin, a protein that enables red blood cells to transport oxygen throughout the body. Both conditions can be fatal.
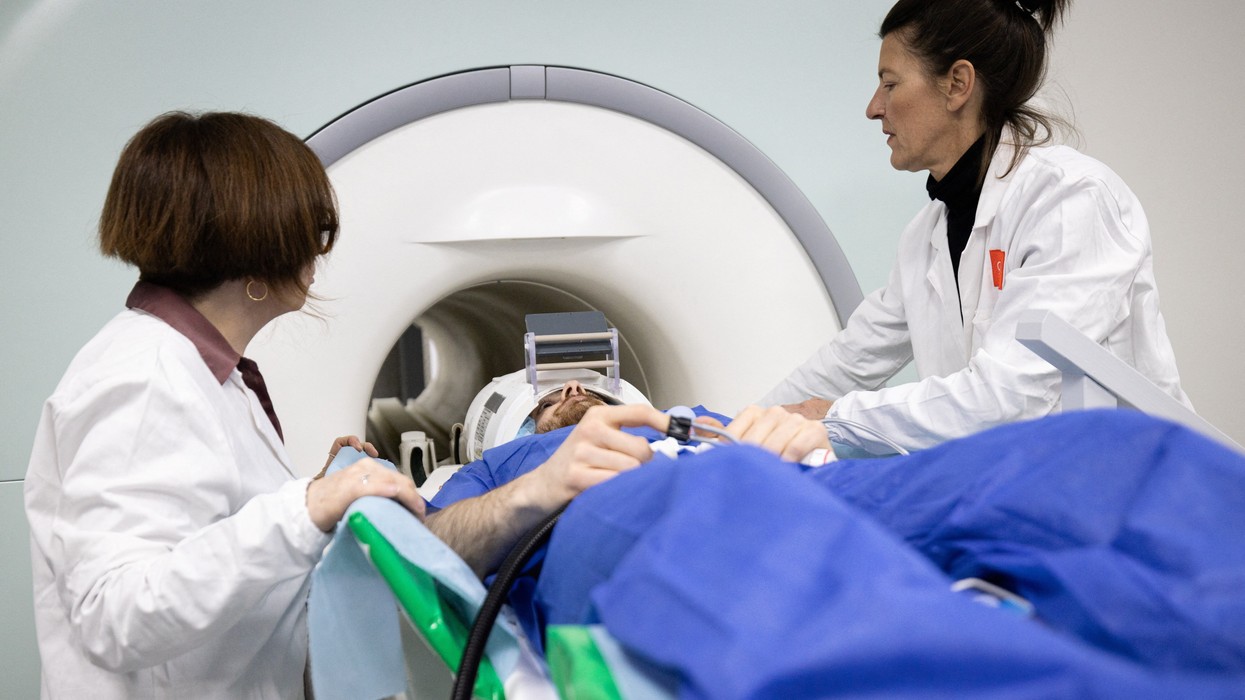
The MHRA said, “Casgevy is designed to work by editing the faulty gene in a patient’s bone marrow stem cells so that the body produces functioning haemoglobin. To do this, stem cells are taken out of bone marrow, edited in a laboratory and then infused back into the patient, after which the results have the potential to be lifelong.”
During this process, laboratory personnel utilise the "genetic scissors" technique to modify, or cut, the DNA of the patient's bone marrow cells, prior to their reintroduction via infusion.
The MHRA has indicated that patients may require a minimum month-long hospital stay while the treated cells establish themselves in the bone marrow and begin production of red blood cells containing the stable form of hemoglobin.
In a clinical trial evaluating Casgevy for sickle cell disease, 28 out of the 29 patients remained free from major pain episodes, which can necessitate hospitalisation, for at least a year following treatment.
Among the 42 beta-thalassemia patients who participated in the clinical trial, 39 did not require any red blood cell transfusions for a minimum of 12 months following Casgevy treatment.
Consultant haematologist at University College hospital London and expert in sickle cell disease, Dr Sara Trompeter said, “Whilst curative treatments may not be suitable for all, gene therapy offers a real chance of cure for those who are not eligible for bone marrow transplants and so we are delighted that it has been approved by MHRA.”






 Crispello was originally launched by Cadbury in the UK in 2012B&M
Crispello was originally launched by Cadbury in the UK in 2012B&M