Highlights:
- NexGen knee implant linked to high failure rates was used in over 10,000 UK operations.
- Concerns flagged as early as 2014; withdrawn from UK market in 2022.
- Hundreds of patients required corrective surgery, with costs running into millions.
- Manufacturer Zimmer Biomet says patient safety is its “top priority” but will not cover revision costs up front.
Implant used despite early warnings
A knee replacement implant used in thousands of NHS operations was known to have a concerning failure rate eight years before it was withdrawn, a BBC File on 4 Investigates report has found.
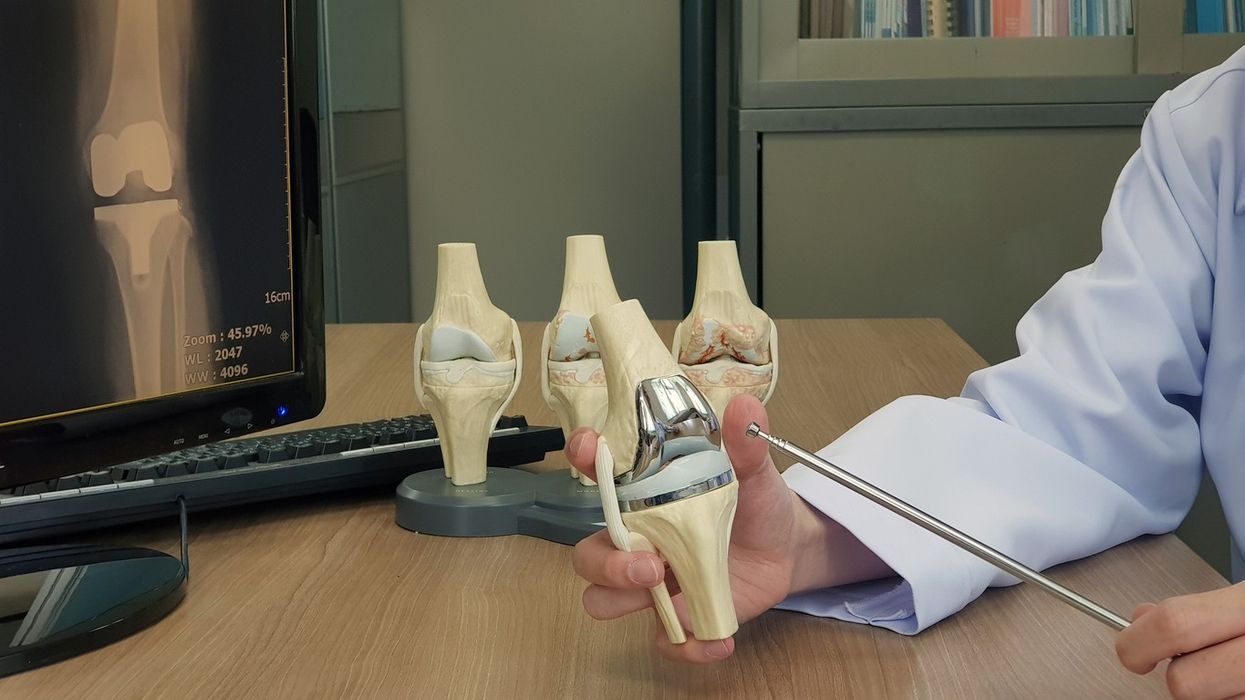
The NexGen implant, made by US manufacturer Zimmer Biomet, was fitted in more than 10,000 patients between 2012 and 2022. Concerns were first raised by the National Joint Registry (NJR) in 2014, though insufficient data at the time made it difficult to draw firm conclusions.
The model in question included a modified “stemmed option tibial component” or “tibial tray”, lacking a layer of plastic found in earlier versions. It was marketed as a cheaper alternative for the NHS.
Patients left in pain and needing further surgery
Patients have reported severe complications after their implants slipped out of place, damaging bone and causing lasting mobility issues.
Debbie Booker, from Southampton, experienced severe pain a year after her 2016 surgery, eventually requiring a second knee replacement. She says the failed implant left her addicted to strong painkillers and caused long-term health problems, including the need for a hip replacement.
Another patient, “Diana”, had her implant fitted in 2021. When it slipped and began wearing away her shin bone, her consultant told her she was “standing on a broken leg”.
Surgeons raised repeated concerns
Irish knee surgeon Prof Eric Masterson reported a surge in corrective surgeries after switching to the NexGen implant in 2012. He says his concerns were dismissed by Zimmer Biomet representatives, a view echoed by NHS surgeons.
UK knee specialist Prof Leila Biant said she and colleagues raised warnings as early as 2017, but the company was slow to engage in evaluating affected patients.
Recall and high revision costs
By 2022, NJR data suggested patients with the NexGen implant were almost twice as likely to require corrective surgery compared with the average knee replacement. Zimmer Biomet recalled unused units from the UK market that year.
Studies have estimated failure rates for the tibial tray component between 6% and 19%. Hundreds of patients have undergone revision surgery, with more expected.
Each corrective procedure costs between £10,000 and £30,000, according to Southampton University’s Prof David Barrett, meaning the total bill is likely to run into millions. Zimmer Biomet has told sales staff it will not cover diagnostic, follow-up, or revision costs up front.
Official responses
Zimmer Biomet says it is “committed to the highest standards of patient safety, quality, and transparency” and acts in line with regulations when new data becomes available.
NHS England has confirmed it is “currently reviewing the case involving Zimmer Biomet NexGen knee implants”.