The global fight against coronavirus just got stronger as the World Health Organization (WHO) has granted emergency approval for the Covid vaccine made by the Chinese state-owned company Sinopharm.
The WHO on Friday said it had validated the "safety, efficacy and quality" of the Sinopharm jab developed by the Beijing Institute of Biological Products. It further added that this addition has "the potential to rapidly accelerate Covid-19 vaccine access for countries seeking to protect health workers and populations at risk".
Sinopharm is the first vaccine to be developed by a non-Western country to get the green light from WHO. It joins other WHO-approved vaccines including the ones made by Pfizer, AstraZeneca, Johnson & Johnson, and Moderna.
The organization said that the vaccine's efficacy for symptomatic and hospitalized cases of Covid-19 was estimated to be 79%. BioNTech/Pfizer and Moderna have an efficacy rate of around 90% or higher, while the AstraZeneca jab is thought to be around 76%.
One of the main advantages of Sinopharm is that it can be stored in a standard refrigerator at 2-8 degrees Celsius, like the AstraZeneca vaccine.
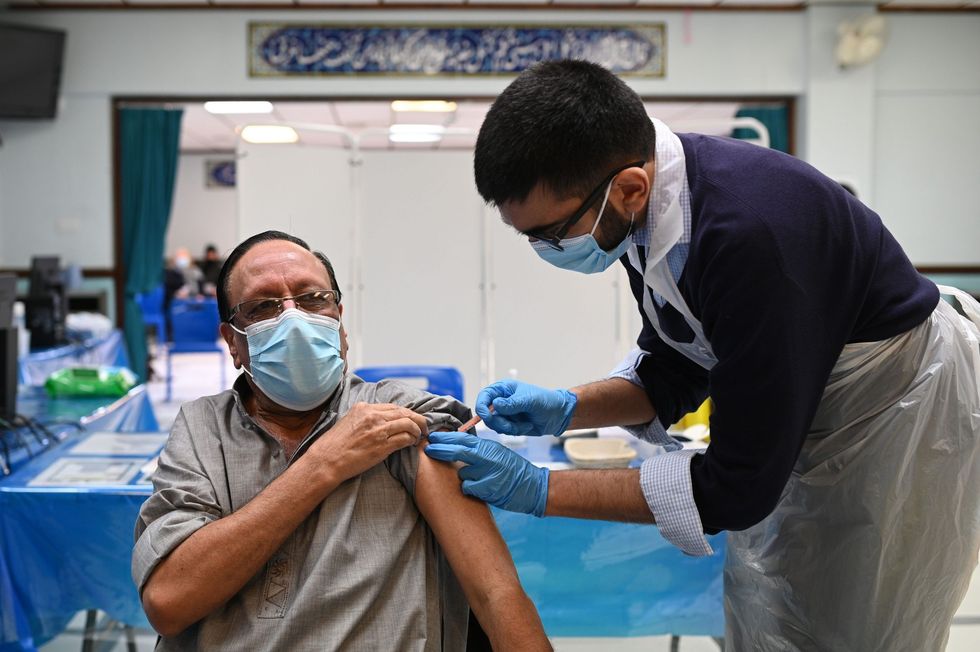
Prior to the approval, an estimated 65 million doses of Sinopharm have already been administered in China and elsewhere as individual health regulators in various countries, including some in Africa, Latin America, and Asia, have approved the Chinese jabs for emergency use. The vaccine is administered in two doses to those aged 18 and over.
WHO’s approval is significant as it indicates that a vaccine is safe and effective. It also means that the vaccine can be used in the global Covax program, which was set up last year to try to ensure fair access to vaccines among rich and poor nations.
The scheme is reportedly struggling with supply problems and this decision of addition of Sinopharm is expected to boost the program.
Another China vaccine developed by Sinovac, along with Russia's Sputnik, is also under assessment by WHO.

















Moglai Bap and Mo Chara of Kneecap perform at Glastonbury Festival at Worthy Farm in Pilton, Somerset, Britain, June 28, 2025. REUTERS/Jaimi Joy
Police may probe anti-Israel comments at Glastonbury
BRITISH police said they were considering whether to launch an investigation after performers at Glastonbury Festival made anti-Israel comments during their shows.
"We are aware of the comments made by acts on the West Holts Stage at Glastonbury Festival this afternoon," Avon and Somerset Police, in western England, said on X late on Saturday (28).
Irish hip-hop group Kneecap and punk duo Bob Vylan made anti-Israeli chants in separate shows on the West Holts stage on Saturday. One of the members of Bob Vylan chanted "Death, death, to the IDF" in a reference to the Israel Defense Forces.
"Video evidence will be assessed by officers to determine whether any offences may have been committed that would require a criminal investigation," the police statement said.
The Israeli Embassy in Britain said it was "deeply disturbed by the inflammatory and hateful rhetoric expressed on stage at the Glastonbury Festival".
Prime minister Keir Starmer said earlier this month it was "not appropriate" for Kneecap to appear at Glastonbury.
The band's frontman Liam Óg Ó hAnnaidh was charged with a terrorism offence last month for allegedly displaying a flag in support of Iran-backed militant group Hezbollah at a concert in November. He has denied the charge.
A British government minister said it was appalling that the anti-Israel chants had been made at Glastonbury, and that the festival's organisers and the BBC broadcaster - which is showing the event - had questions to answer.
Health secretary Wes Streeting said he was also appalled by violence committed by Israeli settlers in the occupied West Bank.
"I'd also say to the Israeli Embassy, get your own house in order in terms of the conduct of your own citizens and the settlers in the West Bank," Streeting told Sky News.
"I wish they'd take the violence of their own citizens towards Palestinians more seriously," he said.
(Reuters)