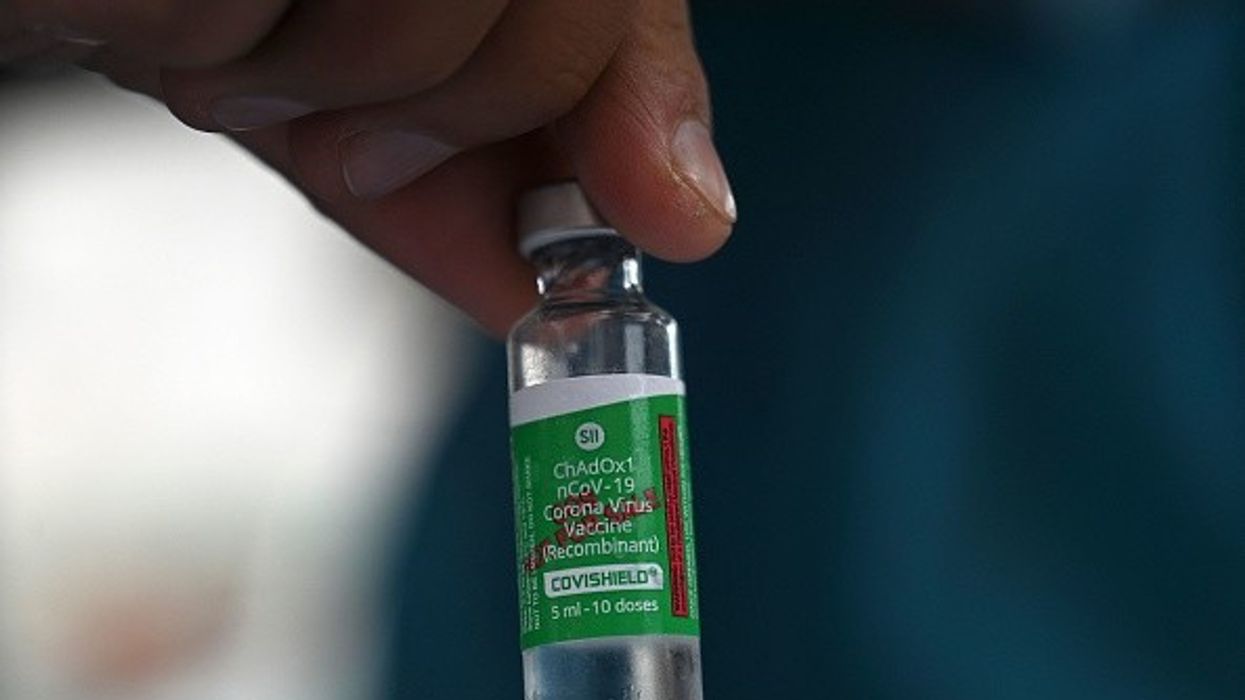
THE Indian drug regulator's subject expert committee on Wednesday (19) recommended full approval for Covishield and Covaxin, the two Covid-19 vaccines that have dominated the country's inoculation drive.
Covishield, developed by the University of Oxford and Anglo-Swedish drugmaker AstraZeneca Plc, is produced in India by the Serum Institute of India. Covaxin, India's first home-grown coronavirus shot, was developed by privately owned Bharat Biotech.
The committee advised that the Drugs Controller General of India upgrade the status of the two shots from restricted use in emergencies for adults, the regulator said on Twitter.
Covaxin and Covishield received emergency use authorisation in India in January 2021, with more than a combined 1.5 billion doses having been administered so far, according to government data.
The Serum Institute has nearly quadrupled its monthly capacity of AstraZeneca's shots to as many as 240 million doses and is prepared to export "large volumes" from January, its CEO told Reuters in October.
(Reuters)